Vaginal Mesh for Stress Urinary Incontinence and Prolapse
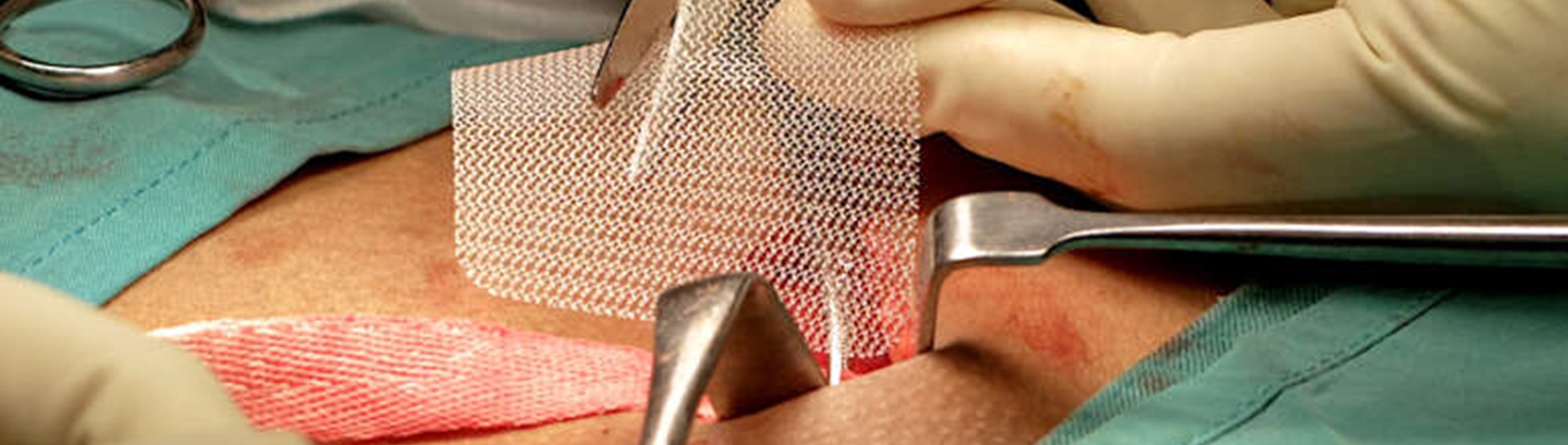
Up until March 2018, transvaginal sling procedures using synthetic, polypropylene mesh have been performed in women by urologists for stress urinary incontinence (SUI) and by also by gynaecologists (for SUI and pelvic organ prolapse - POP). A similar mesh has also been used in other areas of the body e.g. to repair groin hernias, infant umbilical hernias and prolapsed rectum, in both sexes and for some male SUI.
Hospital Episode Statistics (HES) show that 23,742 mesh SUI procedures were performed in the UK between the start of 2014 and the end of 2016: 3,587 (15%) of these were carried out by urologists and 20,155 (85%) by gynaecologists.
Statement to BAUS members following publication of the Cumberlege Report (8 July 2020)
The British Association of Urological Surgeons (BAUS) welcomes the publication of the Independent Medicines and Medical Devices (IMMDS) review report “First Do No Harm” and would like to thank Baroness Julia Cumberlege, Sir Cyril Chantler and Simon Whale, along with all of the members of the review team, for their hard work over the past couple of years.
Many of the Association's members have contributed to the evidence synthesis for the report which has included giving evidence directly to the review team and advising them on matters concerning stress urinary incontinence (SUI) and pelvic organ prolapse (POP) surgery. We are grateful for all of these contributions. The Association is fully supportive of the report’s findings and recommendations and recognises the suffering some women have had to endure as a result of polypropylene mesh placed for stress urinary incontinence and pelvic organ prolapse.
We would like to begin the process of recovery immediately, with a primary objective of regaining the trust of mesh-injured patients. To this end we will pledge to guide our members appropriately with regard to the implementation of the recommendations made in the IMMDS report:
- Apology – the IMMDS report recommends a fulsome apology to mesh-injured patients and BAUS was first of the speciality associations to do this. In 2019, during the IMMDS oral evidence hearing, the BAUS mesh lead, Chris Harding stated “Yes, we do owe women an apology, without doubt, and I would give that unreservedly on behalf of BAUS”
- Patient Safety Commissioner – the report recommends the appointment of a patient safety commissioner to champion the value of listening to patients. BAUS believes that this role should be independent and not allied to any of the professional societies. Nevertheless, BAUS would wholeheartedly support the appointed commissioner on clinical matters where specialist knowledge is required.
- Redress Agency – the review has recommended an independent redress agency be created for those harmed by pelvic mesh to aid with the resolution of disputes between patients and the healthcare system. BAUS is happy to assist this process and would specifically offer topic-specific knowledge as and when required.
- Specialist Mesh Centres – the creation and set-up of specialist mesh centres to provide comprehensive treatment for mesh complications has been recommended and BAUS has been at the heart of this process since its inception. Several BAUS members have assisted NHS England (NHSE) and the Department of Health and Social Care (DHSC) with this workstream and the announcement of these centres is expected in the near future. We believe that there is a high degree of surgical expertise amongst our members and several of them have already accrued significant experience in mesh removal surgery and published their data, in line with the plea for “data-sharing” made by the IMMDS review committee today. The report acknowledged that further research and expert consensus is needed with regard to some of the issues surrounding the treatment of mesh complications and to this end the BAUS mesh lead is liaising with the International Continence Society (ICS) on the creation of a consensus document to be published later in the year. This ICS initiative is currently led by a BAUS member and the committee includes representation from two other members of the Association.
- Pelvic Floor Registry – Baroness Cumberlege has recommended the creation of a central patient-identifiable database and BAUS has again been fully represented and involved in this NHSE-led project at all stages. We have always stood firm on our view that such a registry should include all surgical treatments for SUI and POP to allow for meaningful comparisons to be made and provide sufficient data to aid the process of fully informed consent when a patient is considering a range of potential treatment options. We are hopeful that the National Pelvic Floor Registry will be piloted by the end of this year.
- Transparency of payments made to clinicians – the IMMDS report recommends that the GMC register be expanded to include a list of financial and non-pecuniary interests for all doctors. BAUS will pledge to engage fully with this process which will enhance the degree of transparency related to such matters.
- Creation of a government-led task force – the review’s final and perhaps most important recommendation is that the government set-up a task force to implement their recommendations. We would be very keen for representation on this task force and the Association is completely supportive of the recommendations made and will do everything required to ensure they are put into practice in a rapid and efficient manner.
BAUS would like to confirm that Mr Chris Harding, immediate past Chairman of the Female, Neurological and Urodynamic Urology Executive Committee will continue in his role as BAUS Mesh Lead for the foreseeable future, and the association will prioritise this issue for as long as is necessary. "If any of our membership has any questions or would like clarification on any particular issues they are encouraged to contact the BAUS office directly."
Chris Harding (BAUS Mesh Lead)
Tim O'Brien (BAUS President)
Read the review
Statement to BAUS members regarding NICE SUI Guidelines (April 2019)
BAUS has noted the contents of the NICE guideline linked above, released on 2 April 2019.
There has already been significant concern raised in the media regarding the guideline recommendation that patients should be able to choose between a range of procedures for the surgical treatment of stress urinary incontinence, including the use of mid-urethral mesh sling procedures.
BAUS would like to make its position absolutely clear to the membership.
Although we are strong advocates for patient choice, we would currently advise members to continue to abide by the recommendations set out by the Independent Medicines and Medical Devices review, which were supported by the Department of Health and Social Care in Summer 2018. The pause on mesh surgery for stress incontinence (see below) remains in place, and this will be the case until a series of pre-specified conditions are met.
The publication of the NICE guideline does not change this and we would advise members not to use mesh for the treatment of stress incontinence, in either the NHS or the independent sector, until further notice."
Chris Harding (BAUS Mesh Lead)
Read the NICE guideline (NG123)
Halt on the use of surgical mesh for SUI surgery (from 10 July 2018)
A “period of high vigilance restriction” regarding vaginal mesh surgery was announced by the Cumberlege review, with effect from 10 July 2018.
Statement on the UK Parliament website
BAUS welcomes any recommendations that will help establish the nature and extent of complications suffered by women undergoing these types of surgery. Furthermore, we are keen to support the implementation of processes that will ensure existing patients with complications from mesh surgery are given the opportunity to be managed in centres with the required levels of experience, expertise and resources: click below to access information on this website about these centres.
BSUG statement
BAUS will now engage with NHS England, the Department of Health & Social Care and the Cumberlege review team, via our relevant section leads, to ensure that all conditions set out by the review are met before the lifting of this period of temporary suspension.
Who conducted the review
General details of the review.
Information about mesh removal
Two patient information leaflets have been produced by the NHS in collaboration with the British Society of Urogynaecology (BSUG), the Patient Information Forum, the Pelvic Floor Society and BAUS. These can be found by clicking on the links below:
Mesh removal for vaginal prolapse
Mesh removal for pelvic organ prolapse
Transobturator mesh removal
Retropubic mesh tape removal
Patient Decision Aid for removal of mesh used for incontinence
Treating complications from mesh used for stress urinary incontinence
NHS England Mesh Centres
NHS England and NHS Improvement have commissioning specialised services for women with complications of mesh inserted for urinary incontinence and vaginal prolapse. These hospitals will be leading the multidisciplinary teams (MDTs) providing this specialist service. Regional MDTs will also work together to a national MDT arrangement to ensure that women who have complex needs, that may require specific expertise, can be referred to the centre best placed to manage their care.
The following Trusts are working with NHS England and NHS Improvement, and each other, to provide this service:
- Newcastle Upon Tyne Hospitals NHS Foundation Trust;
- Sheffield Teaching Hospitals NHS Foundation Trust;
- Manchester University NHS Foundation Trust;
- Cambridge University Hospitals NHS Foundation Trust;
- University College London Hospitals NHS Foundation Trust;
- University Hospitals of Leicester NHS Trust;
- Nottingham University Hospitals NHS Trust;
- Southampton General Hospital NHS Trust;
- North Bristol NHS Trust.
Find a Mesh Centre
The table below provides lead urologists for each region, and contact details / addresses for referrals to a specialist mesh centre. Referrals can only be made by your general practitioner (GP) or other registered healthcare professional.
| NHS REGION |
Mesh Centre |
LEAD UROLOGIST |
LEAD URO-GYNAECOLOGIST |
ADDRESS FOR REFERRALS & CORRESPONDENCE |
| North East |
Newcastle upon Tyne NHS Foundation Trust
|
Prof. Chris Harding |
Dr Karen Brown |
Royal Victoria Infirmary, Queen Victoria Road,
Newcastle upon Tyne NE1 4LP |
| Yorkshire |
Sheffield Teaching Hospitals NHS Foundation Trust |
Mr Richard Inman |
Prof. Swati Jha
|
Jessop Wing, Sheffield Teaching Hospitals,
Glossop Road, Sheffield S10 2JF |
| North West |
Manchester University NHS Foundation Trust |
Prof. Ian Pearce |
Dr Karen Ward |
Warrell Unit, St Mary's Hospital
Oxford Road, Manchester M13 9W |
| East Midlands |
University Hospitals of Leicester NHS Trust |
Mr Jaskarn Rai |
Mr Rod Teo |
University Hospitals of Leicester
Gwendolen Road, Leicester LE5 4PW |
| East Midlands |
Nottingham University Hospitals NHS Trust |
Miss Frances Burge |
Mr Paul Hooper |
Nottingham City Hospital, Hucknall Road
Nottingham NG5 1PB |
| East of England |
Cambridge University Hospitals NHS Foundation Trust |
Miss Suzanne Biers |
Mr Ashish Pradhan |
Depts of Urology & Urogynaecology
Addenbrooke's Hospital, Hills Road
Cambridge
CB2 0QQ |
| Greater London |
University College London Hospitals NHS Foundation Trust
|
Miss Tamsin Greenwell |
Miss Tamsin Greenwell |
UCLH Mesh Centre
235 Euston Road, London NW1 2PG |
| South East |
University Hospital Southampton NHS Foundation Trust
|
Miss Melissa Davies |
Mr Ash Monga |
Southampton General Hospital, Tremona Road, Southampton,
Hants SO16 6YD |
| South West |
North Bristol NHS Trust
|
Prof. Hashim Hashim |
Mr Chendrimada Madhu |
Mesh Complications Specialist Service, Bristol Urological Institute, Southmead Hospital, Bristol BS10 5NB |
In Scotland, the centralised Mesh Removal Service in based in Queen Elizabeth University Hospital in Glasgow. The mesh Lead is Miss Karen Guerrero, Urogynaecology Consultant.
Further information available here
Further information on mesh referral service is available here
In Northern Ireland, the complex mesh centre in Belfast (Belfast Health & Social Care Trust) is the national centre for women with mesh complications. This service is located in Belfast Health & Social Care Trust and the leads are Dr Patrick Campbell and Dr Lucia Dolan, Consultant Urogynaecologists.
Further information is available here
BAUS will review & update this table as additional information is received. Southampton and Bristol have provisionally accepted responsibility for mesh removal referrals in the South East & South West respectively.
Further information
Pelvic Floor Registry
The Pelvic Floor Registry Developed by NHS Digital is now being piloted. The aim is to monitor and improve patient safety. It records the surgical mesh implants, and related medical devices, given to patients, and the organisations and surgeons that have carried out the procedures.
Further information available here
RCOG training on mesh
Use our feedback form & send us your comments