Vaginal Mesh for Stress Urinary Incontinence: Information for Healthcare Practitioners
01 March 2015
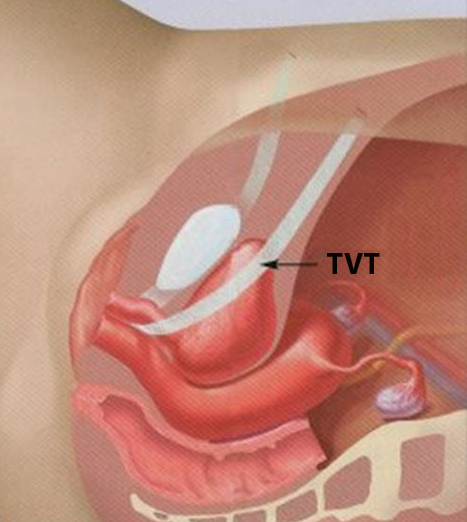
Reporting device complications to the MHRA
There have been significant concerns about the safety of synthetic meshes for prolapse and incontinence surgery.
To date the MHRA have received few reports of adverse events from medical practitioners.
The MHRA have now produced A Guide to Reporting Device Complications, with specific reference to synthetic meshes used for vaginal prolapse and for incontinence surgery.
View other news